The following example shows how to build a project in which the detailed microkinetic of an heterogeneous catalytic reactor is considered by including adsorption and desorption of compounds over the catalyst surface. Also, the equivalent formulation using LHHW kinetics is developed.
Consider a catalytic reactor in which the gaseous reactive
A is firstly adsorbed on the catalyst surface, represented by reaction
R1:
Where
S represent the free catalyst surface, and
AS is the adsorbed
A on the catalyst surface.
When Partial Pressure was defined as the
Concentration in the
Units Configuration node, the net rate of reaction
R1 is given by:
Where
PA is the partial pressure of
A in the gas phase,
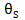
and
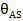
represent the free surface fraction and the surface fraction occupied by the adsorbed compound
A, respectively; and
k1F and
k1R are the forward and reverse kinetic constants.
The reaction that takes place in the surface is given by the
R2 reaction:
For which the net reaction rate is given by:
Finally, the adsorbed product
B is desorbed from the catalyst surface, as represented by reaction
R3:
The previous reaction net rate is given by:
where
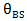
represents the catalyst surface fraction occupied by the adsorbed compound
B, and
PB is the partial pressure of the product.
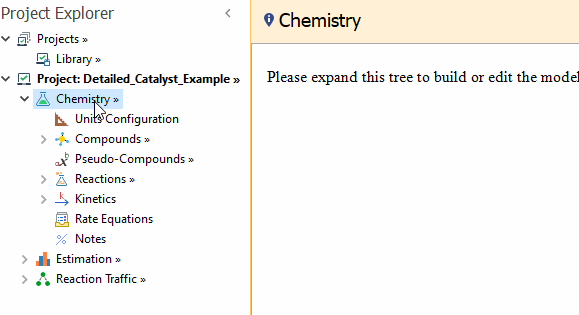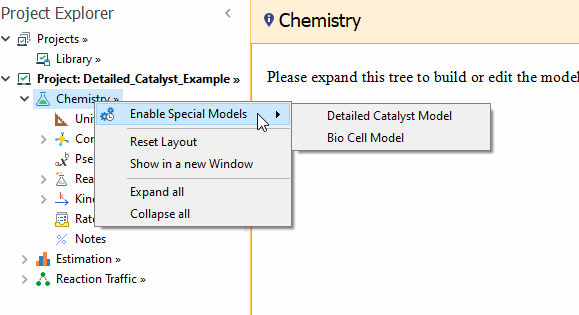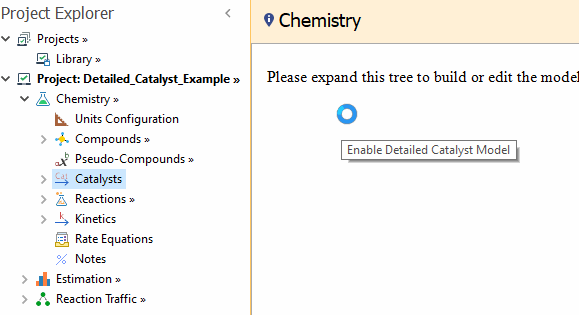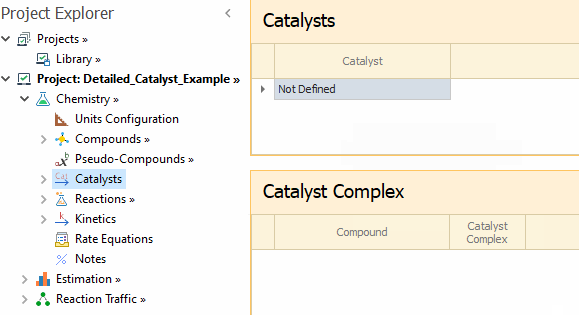
To implement this model in REX, you have to use the
Enable Special Models → Detailed Catalyst Model action available in the right click menu of the
Chemistry node:
After that, the
Catalysts child node is shown in the Chemistry tree.
Now we define
Catalyst Mass as the Rate Basis in the
Units Configuration node . Also,
Partial Pressure is chosen as the Concentration option.
Next we enter all species involved in the
Compounds node: for the example,
A and
B compounds, plus the catalyst
S and the adsorbed species
A-S and
B-S.
After entering the compounds, you can select
S as the
Catalyst, and the adsorbed species on the catalyst surface as
Catalyst Complex in the
Catalysts node:
By doing the previous, REX takes into account the surface fractions
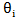
when defining the rate orders in the
Parameters node, if
i was defined as either Catalyst or Catalyst Complex.
Also, the sum of the surface fractions equal to 1 is imposed: for this example, that constraint is represented by:
In the
Reactions node, the three reactions described above are entered:
In the
Kinetics node the forward directions must be included for all the reactions, together with the reverse direction for both adsorption and desorption reactions. The
Initialize Orders action is performed in the
Parameters node, and the rate constant values considered for this example are the following:
From the folder Optience Corporation\REX Suite\Examples you can import the Detailed_Catalyst_Example.rex file, in which a PFR reactor has been considered and simulation results are included.
The previous reaction system can alternatively be modeled by using the LHHW kinetics.
In order to convert the numerical values from the previous Detailed Catalysis project, the pseudo steady state hypothesis is posed for the catalyst complexes, thus the net rate for each of them is set to zero:
From the previous equations it can be obtained:
Replacing
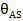
into
rR2:
After replacing
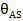
and
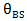
into the sum of all fractions equal to one, solving for
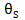
and replacing into the last equation it is obtained:
You can then build a project using LHHW kinetics and loading the parameters obtained by converting the ones used in the Detailed Catalysis Model. The
Kinetics - LHHW Case example could help you in order to define the site for the LHHW project.
Also, you can import from the
Optience Corporation\Rex Suite\Examples folder the Equivalent_LHHW_Example.rex file, in which the LHHW-equivalent model was built.
You can compare it to the Detailed_Catalyst_Example.rex example.
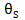 and
and 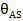 represent the free surface fraction and the surface fraction occupied by the adsorbed compound A, respectively; and k1F and k1R are the forward and reverse kinetic constants.
represent the free surface fraction and the surface fraction occupied by the adsorbed compound A, respectively; and k1F and k1R are the forward and reverse kinetic constants.
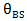 represents the catalyst surface fraction occupied by the adsorbed compound B, and PB is the partial pressure of the product.
represents the catalyst surface fraction occupied by the adsorbed compound B, and PB is the partial pressure of the product.
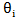 when defining the rate orders in the Parameters node, if i was defined as either Catalyst or Catalyst Complex.
when defining the rate orders in the Parameters node, if i was defined as either Catalyst or Catalyst Complex. 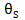 and
and 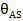 represent the free surface fraction and the surface fraction occupied by the adsorbed compound A, respectively; and k1F and k1R are the forward and reverse kinetic constants.
represent the free surface fraction and the surface fraction occupied by the adsorbed compound A, respectively; and k1F and k1R are the forward and reverse kinetic constants.
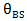 represents the catalyst surface fraction occupied by the adsorbed compound B, and PB is the partial pressure of the product.
represents the catalyst surface fraction occupied by the adsorbed compound B, and PB is the partial pressure of the product.
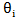 when defining the rate orders in the Parameters node, if i was defined as either Catalyst or Catalyst Complex.
when defining the rate orders in the Parameters node, if i was defined as either Catalyst or Catalyst Complex. 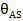 into
rR2:
into
rR2:
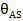 and
and 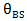 into the sum of all fractions equal to one, solving for
into the sum of all fractions equal to one, solving for 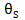 and replacing into the last equation it is obtained:
and replacing into the last equation it is obtained:
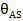 into
rR2:
into
rR2:
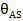 and
and 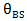 into the sum of all fractions equal to one, solving for
into the sum of all fractions equal to one, solving for 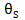 and replacing into the last equation it is obtained:
and replacing into the last equation it is obtained: